|
Ningbo Mingzhi Stainless Product Co.,Ltd
|
Stainless steel strip suppliers Stainless steel coil suppliers
| Price: | 10000.0~11000.0 USD |
| Payment Terms: | T/T,L/C,D/A,D/P, |
| Place of Origin: | Zhejiang, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
Product Detail
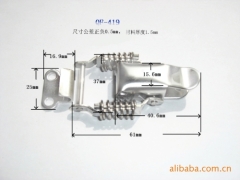
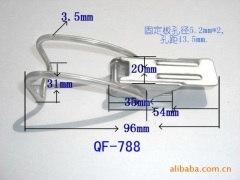
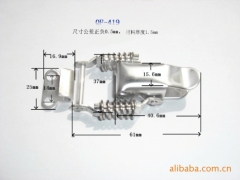
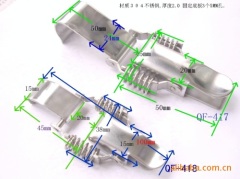
The characteristics and application of 409 stainless steel:
409 stainless steel, the most inexpensive models (England), usually used in automobile
Five, corrosion-resistant stainless steel why?
All metals and the reaction of oxygen in the atmosphere, the formation of oxide film on the surface. Unfortunately, the iron oxide in the form of ordinary carbon steel on oxidation, rust continues to expand, eventually forming holes. You can use paint or oxidation-resistant metal (for example, zinc, nickel and chromium) plating to ensure the carbon steel surface, but, as everyone knows, this protection is only a thin film. If the layer is destroyed, the following steel began to rust.
Stainless steel corrosion resistance depends on the chrome, but because the chromium steel is one of the components, so the protection method is not the same as.
In addition to 10.5% chromium, the atmospheric corrosion resistance of steel increased significantly, but the chromium content is higher, although still can improve corrosion resistance, but not obvious. The reason is the steel with chromium alloying treatment, the surface oxide type change into similar surface pure chromium metal oxide formed on the. This close adhesion chromium oxide protection surface, prevent further oxidation. The oxide layer is very thin, through which you can see the natural luster of steel surface, the stainless steel has a unique surface. And, if the damaged surface, the exposed steel surface and atmospheric reaction for self repair, to form the "film", continue to play a protective role.
Therefore, all stainless steel has a common characteristic, namely the chrome content in 10.5% above.
Related Search
Stainless Steel Coil Strip
Coil Stainless Steel
Stainless Steel Coil
Stainless Steel In Coil
Stainless Steel Coil Sheet
Polished Stainless Steel Coil
More>>