|
Ningbo Mingzhi Stainless Product Co.,Ltd
|
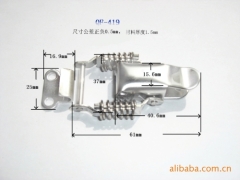
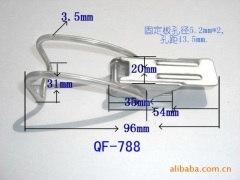
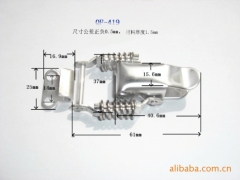
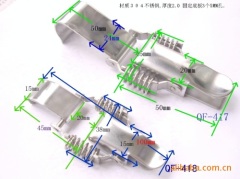
China Stainless Steel Strapping Band China Stainless steel wire
| Price: | 10000.0~11000.0 USD |
| Payment Terms: | T/T,L/C,D/A,D/P, |
| Place of Origin: | Zhejiang, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
Product Detail
The characteristics and application of 409 stainless steel:
409 stainless steel, the most inexpensive models (England), usually used in automobile
A brief introduction, stainless steel
All metals and the reaction of oxygen in the atmosphere, the formation of oxide film on the surface. Unfortunately, the iron oxide in the form of ordinary carbon steel on oxidation, rust continues to expand, eventually forming holes. You can use paint or oxidation-resistant metal (for example, zinc, nickel and chromium) plating to ensure the carbon steel surface, but, as everyone knows, this protection is only a thin film. If the layer is destroyed, the following steel began to rust
The resistance of air, steam, water and other corrosive medium and weak acid, alkali, salt and other chemical etching medium corrosion of steel. Also known as stainless acid-resisting steel. In practical applications, often weak corrosion resistant steel, stainless steel, the corrosion of the steel is called acid-resisting steel. Due to differences in the chemical composition of the two, the former is not resistant to chemical corrosion, and the latter is normally has no rust. The corrosion resistance of stainless steel alloy elements on the contained in steel. Chrome is made of stainless steel corrosion resistance for the basic elements, when the Cr content in steel is 1.2% or so, oxygen effect of chromium and corrosive medium, the formation of a thin layer of oxide film on the steel surface (since the passivation film), the matrix can prevent further corrosion of steel. In addition to chromium, common alloying elements are nickel, molybdenum, titanium, niobium, copper, nitrogen and so on, to meet a variety of uses and properties of stainless steel requirements.
Related Search
Stainless Steel Band
Stainless Steel Band Watch
Stainless Steel Wire Mesh
Stainless Steel Wire
304l Stainless Steel Wire
Galvanized Stainless Steel Wire
More>>